CURABLE
curable Inc. started as a genetic analysis and genetic disease diagnosis project in 2000 and is focusing on research using Korean epigenetic data based on a cohort selected as an academic service company of the Korea Centers for Disease Control and Prevention in 2016.
In 2021, we developed DDS technology using new microfluidic-based lipid nanoparticles (LNPs) to become a reliable partner for biopharmaceutical developers, and is set to launch in the fourth quarter of 2022 primarily by preparing RNA treatment and vaccine consignment production services.
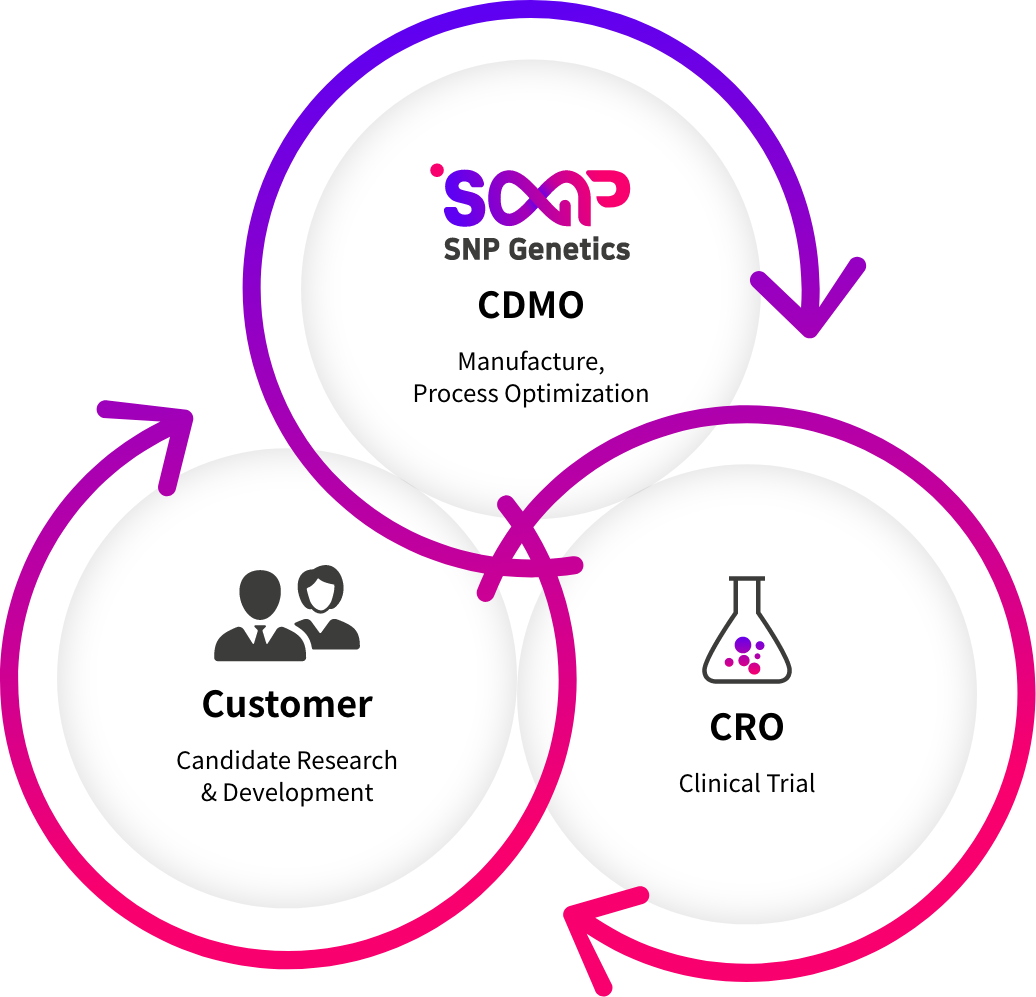
We provide customization services from research to non-clinical and clinical trial samples at cGMP-certified facilities. In addition to consignment production, we will expand our business to consignment production that provides drug development and manufacturing services at once, and we will work with customers who need mass production of small molecule new drugs and medicines as well as RNA.
Our Offerings
Customized Manufacturing Services

RNA Synthesis Service
- non-GMP grade research RNA (miRNA, siRNA, ASO, mRNA)
- cGMP grade preclinical RNA: min. 10μmol
- cGMP grade clinical RNA: min. 10μmol
* Sample yield may change according to list of analysis required

Customized DDS Synthesis Service
- Customer provided RNA + Custom DDS
(LNP, Liposome, PLGA, GalNAc): max. 5g/batch (1000mL/min)
- Custom RNA + Custom DDS: max. 5g/batch (1000mL/min)
*Capacity based on LNP formulation
Our Abilities
RNA based therapeutics & vaccines contract manufacturing services applying DDS manufacture technology and nano particle mass produce technology.
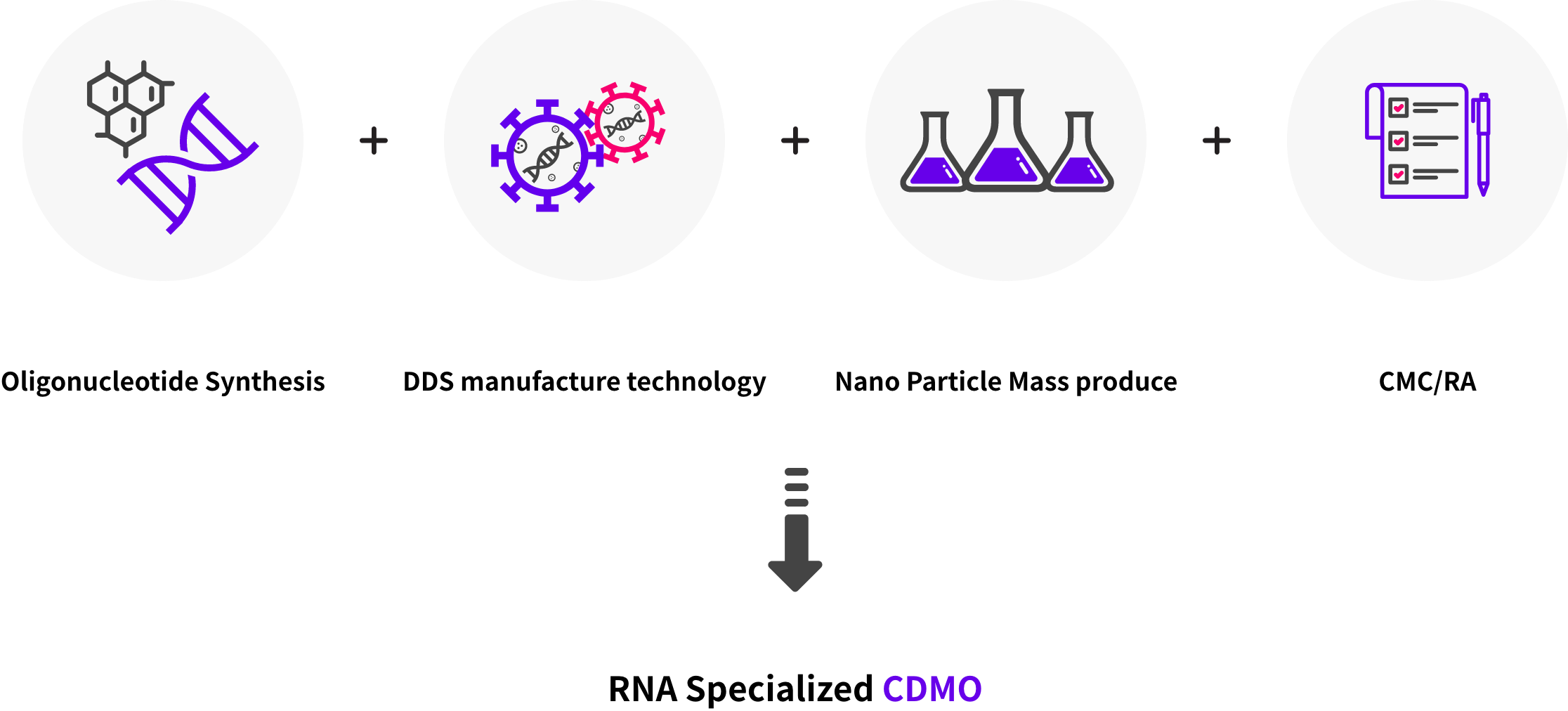
- Microfluidics platform solved problems in nano particle mass production.
- New LNP manufacturing technology solved existing LNP patent problem.
- CMC/RA Solution fullfill IND & NDA regulation requirements